Quick Summary : What You Need to Know: Mitochondrial diseases are genetic disorders that impair…
- August 26, 2024
- Comments Off on Understanding the Genetic Basis of Mitochondrial Disease
The Complex World of Mitochondrial Disease
Mitochondrial disease is a group of disorders caused by dysfunctional mitochondria, the tiny powerhouses within our cells that generate the energy needed for our bodies to function. These disorders are often genetic, meaning they are passed down from one generation to the next, making them an inherited condition.
Understanding the genetic basis of mitochondrial disease is crucial for grasping how these conditions develop, how they affect individuals, and what potential treatments might look like in the future.
What are Mitochondria and Why Do They Matter?
To understand mitochondrial disease, it’s essential first to understand the role of mitochondria in the body. Mitochondria are tiny organelles found in almost every cell in the body, with the primary function of producing energy in the form of adenosine triphosphate (ATP). ATP is often referred to as the energy currency of the cell, and it powers various cellular processes that are critical for survival.
When mitochondria are not functioning correctly, cells don’t get the energy they need to perform their normal functions. This energy deficiency can lead to a variety of symptoms, depending on which cells and tissues are most affected. Organs that require a lot of energy, such as the brain, heart, muscles, and liver, are often the most impacted by mitochondrial disease.
The Role of Genetics in Mitochondrial Disease
Mitochondrial disease is primarily a genetic condition, meaning it arises due to mutations in the genes that are involved in mitochondrial function. These mutations can occur in two types of DNA: nuclear DNA (nDNA) and mitochondrial DNA (mtDNA).
Nuclear DNA (nDNA) and Mitochondrial DNA (mtDNA)
Nuclear DNA (nDNA): Most of our genetic material is found in the nucleus of the cell, where nuclear DNA is housed. This DNA is inherited from both parents, and it contains the vast majority of our genetic information, including genes that code for proteins involved in mitochondrial function.
Mitochondrial DNA (mtDNA): Unlike nuclear DNA, mitochondrial DNA is found in the mitochondria themselves. mtDNA is inherited exclusively from the mother, and it contains a smaller number of genes, but these genes are essential for the mitochondria’s energy-producing functions.
Mutations in either nDNA or mtDNA can lead to mitochondrial disease, but the inheritance patterns and the effects on the body can differ depending on which type of DNA is involved.
Inheritance Patterns of Mitochondrial Disease
Understanding how mitochondrial disease is inherited can be complex due to the involvement of both nDNA and mtDNA. Here are the most common inheritance patterns:
Maternal Inheritance: Since mitochondrial DNA is passed down from mother to child, mitochondrial diseases caused by mtDNA mutations are inherited maternally. This means that if a mother has a mutation in her mtDNA, all of her children will inherit this mutation.
Autosomal Recessive Inheritance: Some mitochondrial diseases are caused by mutations in nDNA and follow an autosomal recessive pattern. In this case, a child must inherit two copies of the mutated gene—one from each parent—to develop the disease. If both parents are carriers, there is a 25% chance with each pregnancy that the child will have the disease.
Autosomal Dominant Inheritance: Less commonly, mutations in nDNA that cause mitochondrial disease can follow an autosomal dominant pattern, where only one copy of the mutated gene is needed for the disease to manifest. If a parent has the disease, there is a 50% chance it will be passed on to their child.
Sporadic Mutations: Sometimes, mitochondrial disease can occur due to new mutations that happen spontaneously in an individual’s DNA, rather than being inherited from a parent. These are called sporadic mutations and can happen in either nDNA or mtDNA.
For more detailed information on these inheritance patterns, the United Mitochondrial Disease Foundation provides an excellent resource.
How Do Genetic Mutations Lead to Mitochondrial Disease?
The genetic mutations that cause mitochondrial disease disrupt the normal functioning of the mitochondria, leading to reduced energy production within cells. This energy deficiency can cause a wide range of symptoms, depending on which cells and tissues are most affected. Here’s how different types of mutations can impact mitochondrial function:
mtDNA Mutations
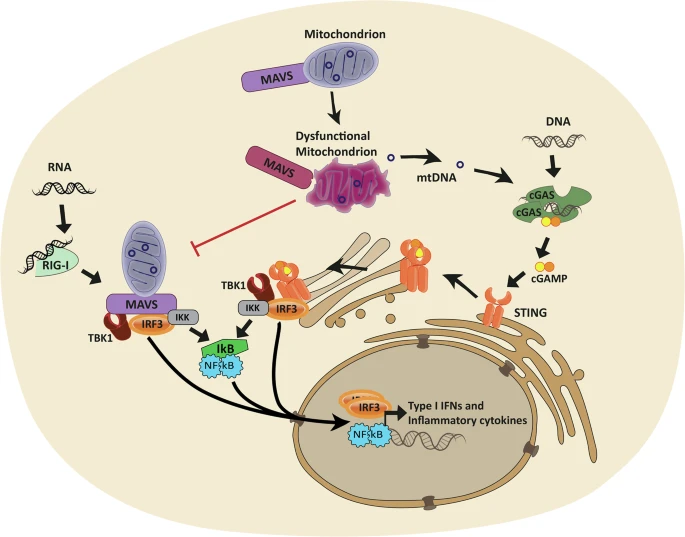
Mutations in mtDNA can directly affect the proteins that are involved in the electron transport chain, the series of steps that mitochondria use to produce ATP. When these proteins are faulty, the mitochondria can’t produce enough energy, leading to cellular dysfunction and the symptoms of mitochondrial disease. Because mtDNA is inherited maternally, these diseases often have a distinct pattern of inheritance within families.
nDNA Mutations
Mutations in nDNA can affect a wide variety of cellular processes that are important for mitochondrial function, including the replication and repair of mtDNA, the assembly of the electron transport chain, and the transport of molecules into and out of the mitochondria. These mutations can lead to a loss of mitochondrial function and energy production, resulting in the symptoms of mitochondrial disease.
Diagnosing Mitochondrial Disease
Diagnosing mitochondrial disease is often a complex process due to the wide variety of symptoms and the genetic diversity of the condition. Diagnosis typically involves a combination of clinical evaluation, genetic testing, and sometimes a muscle biopsy to assess mitochondrial function directly.
Clinical Evaluation
The first step in diagnosing mitochondrial disease is usually a thorough clinical evaluation, where a doctor will assess the patient’s symptoms, medical history, and family history. Because mitochondrial disease can affect many different organs and systems, doctors may look for a pattern of symptoms that suggest a mitochondrial disorder.
Genetic Testing
Genetic testing plays a crucial role in diagnosing mitochondrial disease. By analyzing a patient’s DNA, doctors can identify mutations in mtDNA or nDNA that are known to cause mitochondrial disease. Genetic testing can also help determine the inheritance pattern of the disease, which is important for family planning and understanding the risk to other family members.
Muscle Biopsy
In some cases, a muscle biopsy may be performed to assess mitochondrial function directly. During this procedure, a small sample of muscle tissue is taken and analyzed under a microscope. This can reveal abnormalities in the structure and function of the mitochondria, providing further evidence of mitochondrial disease.
For more details on diagnostic procedures, you can refer to the National Institute of Neurological Disorders and Stroke.
Living with Mitochondrial Disease
Living with mitochondrial disease can be incredibly challenging, not only for the patient but also for their families. The symptoms of mitochondrial disease can vary widely in severity, and the progressive nature of the condition means that symptoms often worsen over time.
Managing Symptoms
There is currently no cure for mitochondrial disease, so treatment is focused on managing symptoms and improving quality of life. This often involves a multidisciplinary approach, with doctors from various specialties working together to address the patient’s needs.
Medications: Patients may be prescribed medications to manage specific symptoms, such as seizures, muscle pain, or heart problems.
Physical Therapy: Physical therapy can help maintain muscle strength and mobility, improving the patient’s ability to carry out daily activities.
Dietary Supplements: Some patients may benefit from dietary supplements that support mitochondrial function, such as coenzyme Q10, L-carnitine, or riboflavin.
Emotional and Psychological Support
The emotional and psychological impact of living with mitochondrial disease can be profound. Patients and their families may experience anxiety, depression, and feelings of isolation. Access to counseling, support groups, and mental health services is crucial for helping families cope with the challenges of the disease.
Family Planning and Genetic Counseling
For families affected by mitochondrial disease, genetic counseling can provide valuable information about the risks of passing the condition on to future generations. Genetic counselors can help families understand the inheritance patterns of the disease and discuss options such as preimplantation genetic diagnosis (PGD) if they are planning to have children.
The Future of Mitochondrial Disease Research
Research into mitochondrial disease is ongoing, and there is hope that new treatments and therapies will be developed in the future. Scientists are exploring a variety of approaches to treat mitochondrial disease, including gene therapy, stem cell therapy, and targeted drug therapies.
Gene Therapy
Gene therapy is a promising area of research that involves correcting the genetic mutations that cause mitochondrial disease. By delivering healthy copies of the mutated genes to the affected cells, gene therapy has the potential to restore normal mitochondrial function and prevent the symptoms of the disease.
Stem Cell Therapy
Stem cell therapy is another exciting area of research. This approach involves using stem cells to replace or repair damaged cells in the body, potentially restoring normal mitochondrial function. While still in the experimental stages, stem cell therapy offers hope for future treatments.
Targeted Drug Therapies
Researchers are also working to develop drugs that specifically target the defective mitochondria in patients with mitochondrial disease. These drugs aim to improve mitochondrial function and reduce the symptoms of the disease, providing a new avenue for treatment.
For updates on the latest research in mitochondrial disease, consider following resources like MitoAction, an organization dedicated to supporting those affected by mitochondrial disease.
Conclusion
Mitochondrial disease is a complex and challenging condition rooted in genetic mutations that affect the body’s ability to produce energy. By understanding the genetic basis of mitochondrial disease, we can better appreciate the impact it has on individuals and their families, and why ongoing research is so critical.
With advancements in genetic research, gene therapy, and other innovative treatments on the horizon, there is hope for improving the lives of those affected by this devastating disease.
The Liam Foundation is committed to supporting these efforts and helping families navigate their journey with mitochondrial disease. Together, we can bring hope and work towards a brighter future.
Share This:
The Liam Foundation
Related Posts
The COVID-19 pandemic, caused by the SARS-CoV-2 virus, has led to significant global health challenges.…
A groundbreaking clinical trial led by the Research Institute of the McGill University Health Centre…
Mitochondrial DNA Depletion Syndrome (MTDPS) encompasses a group of rare genetic disorders characterized by a…
The Liam Foundation stands as a beacon of hope for families grappling with the challenges…
Mitochondrial disease treatments have long been a challenging frontier in medical research. As we delve…
Mitochondrial disease symptoms are complex disorders that can affect people of all ages, from infants…
To appreciate the significance of recent Mitochondrial disease research advances, it’s essential to understand the…
Understanding the Genetic Basis of Mitochondrial Disease, Mitochondrial disease is a group of disorders caused…
Mitochondrial disease is a group of disorders caused by dysfunctional mitochondria, the parts of the…